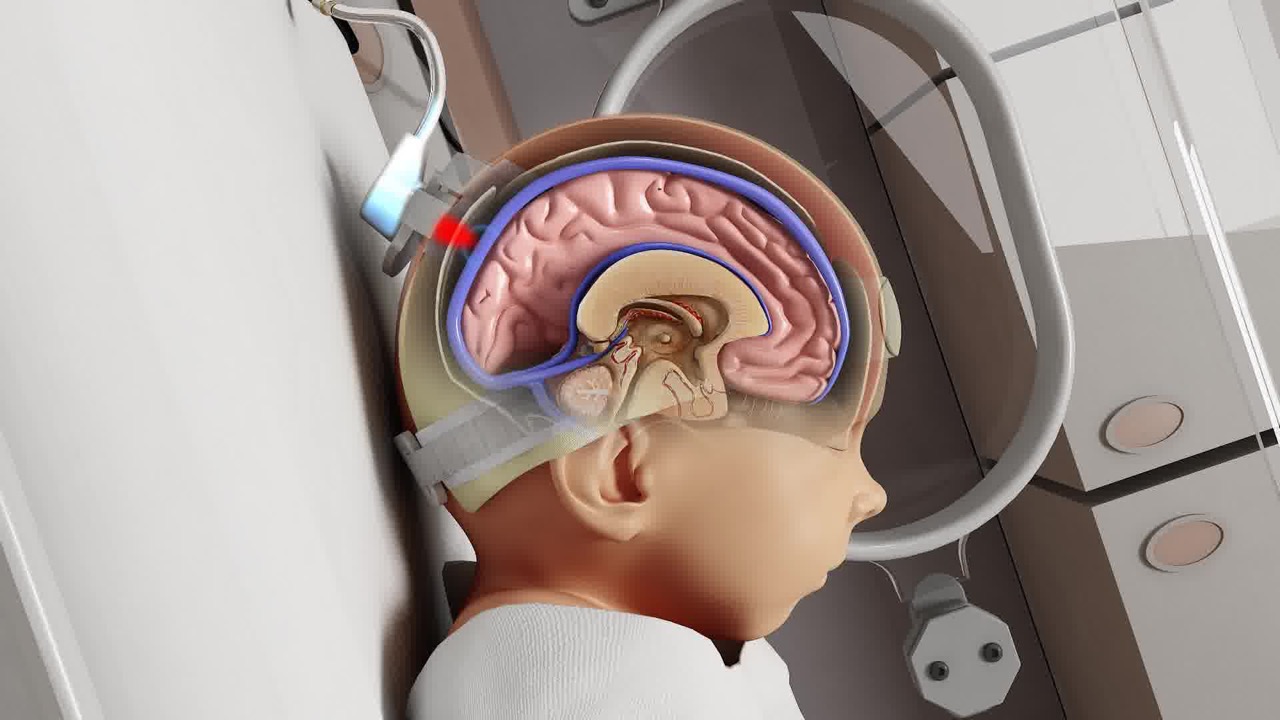
Houston medical device startup Noninvasix, Inc., developers of a noninvasive brain oxygenation monitor for the NICU, is collaborating with the Consortium of Technology and Innovation in Pediatrics (CTIP) to accelerate the development of its advanced prototype, navigate the FDA regulatory process and identify strategic paths to market.
Designed using optoacoustic technology to noninvasively monitor brain oxygenation levels in preterm and low birth-weight babies, Noninvasix’s system aims to reduce hypoxic ischemic encephalopathy—type of brain damage that occurs when an infant’s brain doesn’t receive enough oxygen and blood – which is responsible for 23% of all neonatal deaths and is the precursor for cerebral palsy.
“The need for infant care solutions, especially those designed for the NICU, are more important than ever as the rate of preterm births increases,” said Graham Randall, Ph.D., president and CEO of Noninvasix. “Promising preliminary data, along with a partnership with CTIP, will help us fast track our optoacoustic technology from trials to the marketplace, where it is needed most.”
CTIP, one of seven national consortia funded by the Food and Drug Administration’s Pediatric Device Consortia program, established at Children’s Hospital Los Angeles in collaboration with the University of Southern California and Texas Children’s Hospital, will add the cerebral brain monitor to its growing device portfolio, and support Noninvasix through FDA 510(k) clearance and the rigorous commercialization process.
ABOUT NONINVASIX, INC.
Using patented optoacoustic technology, Galveston, Texas-based Noninvasix, Inc. has developed a solution for the safe, accurate and noninvasive monitoring of cerebral oxygenation monitoring in low birth weight and preterm infants. Noninvasix’s undertaking to significantly improve outcomes for preterm infants, while reducing malpractice lawsuits and premiums, is backed by 15 years of optoacoustic research and more than $6.5 million in research grants. For more information, visit www.noninvasix.com.